Quality Management System
Nuclear Medicine
Introduction to Quality Management Systems
The adoption of a quality management system (QMS) should be a strategic decision of a Nuclear Medicine service. Its design and implementation will be influenced by various factors, for example the nature of the provided services, the employed processes as well as the size and structure of the nuclear medicine services. A proper QMS enables the Nuclear Medicine service to achieve certain expectations in its quality policy and helps to ensure satisfaction for the patients. The QMS standardizes the processes to guarantee consistency in providing high level services to patients, referring physicians and other stakeholders in a safe environment.
A quality management system has three main components: quality assurance (QA), quality improvement and quality control (QC). QA is a systematic programme for monitoring and evaluation of the service, helps to ensure reliability, and can help to minimize the uncertainties and errors in equipment performance. QA can also help to identify and rectify problems, errors and malfunctioning earlier. A QA programme in Nuclear Medicine involves all aspects of Nuclear Medicine, including minimizing the exposure to personnel, patients and the public as well as preparation, safety, sterility and administration of radiopharmaceuticals, patient handling, and ensuring the diagnostic quality of images.
Quality control is the process by which the performance level of a service is measured and then compared against the existing standards or tolerance values. Therefore, QC activities are a subset of QA activities, as QA focuses on the processes while QC focuses on the service. Quality control regarding imaging systems may entail a series of performance measurements to assess the quality of the imaging system, the keeping of records of measurements, the monitoring of the accuracy and precision of the results, and corrective actions in case the performance measurements are outside the tolerance levels. Tolerance levels define the range within which the results are acceptable while action levels define the range beyond which a corrective action is required. If the performance of the system is just outside the tolerance range, an immediate corrective action may not always be needed and the imaging system can still be used for patient scanning, but a close monitoring of the system performance is critical in the following tests.
Phantoms are indispensable tools for QC measurements. They are utilized to evaluate diagnostic imaging systemsand provide utility in the areas of radiation protection, radiobiology and radiotherapy. The phantoms can be hot (containing a known amount of radioactivity) or cold (containing no radioactivity) for primarily measuring radiation interaction.
Information about quality management systems and their application in clinical practice is provided in the IAEA Human Health Series No.33 “Quality Management Audits in Nuclear Medicine Practices”. In addition, the IAEA book on Nuclear Medicine Physics also covers the topic of QMS in Nuclear Medicine as well as the topic of Nuclear Medicine in general.
Acceptance tests
Acceptance testing is performed after the arrival of new equipment in order to determine whether the system performs according to the manufacturers specifications and is free of any deficiencies, flaws or defects. Baseline performance of the equipment will also be established at this stage, which provides guidance in the determination of the optimal operating parameters for routine use and ensures that the equipment meets regulatory requirements for radiation safety.
Important principles
Usually, the acceptance testing is performed by the user of the equipment itself or by a third party, for example a qualified medical physicist. These tests usually require sophisticated phantoms and dedicated software to calculate the performance parameters. During the acceptance testing the user should also conduct reference tests which reflect the performance of the system under clinical settings and are usually easy to perform.
Several issues need to be considered before starting acceptance testing.. An accurate dose calibrator is an essential tool for acceptance testing and needs to be available. In addition, the required amount of radioactivity has to be arranged and the system needs to be calibrated properly before starting acceptance testing to avoid interruptions during the testing procedure. Some phantoms are used primarily during acceptance testing and after major repair, therefore the manufacturer may need sufficient notification to ensure these phantoms are available during the acceptance testing. The slit phantom used for testing linearity and intrinsic resolution of gamma cameras is a typical example. In addition to these considerations, the calculation of performance parameters from the image data in PET, SPECT and gamma cameras may require sophisticated software that requires coordination with the manufacturer.
During the acceptance testing, most of the performance parameters of the system should be tested and compared with the manufacturer’s specifications. If any of the test results do not meet the specifications, the analyses should be re-evaluated, and the test should be performed again with close attention to possible mistakes made during the acquisition and processing of the data. If the problem persists, the engineer should be notified to rectify the problem and resolve the issue. After the repair, all required calibrations need to be performed again and the acceptance testing can be repeated.
The IAEA book on Nuclear Medicine Physics provides a comprehensive overview to the topic of Nuclear Medicine in general and also pays close attention to the subject of quality management systems and acceptance testing.
Quality Control in Nuclear Medicine
In the field of Nuclear Medicine, and other specialities based on advanced technology, the attainment of high standards and reliability is of great importance. Therefore, proper quality management systems need to be implemented in the practice of Nuclear Medicine facilities. Quality control (QC), as an important part of a quality management system, utilizes specific measures to ensure the equipment operates within certain quality standards.
Important principles
Quality control of Nuclear Medicine instruments should be undertaken as an integral part of the work of the Nuclear Medicine unit and the members of the unit staff whenever possible. The starting point of QC for each instrument is the selection and acquisition of the instrument itself. The choice of an appropriate site for installation of the instrument should be considered within the scope of quality control, as it may affect performance. When an instrument is received and installed, a series of acceptance tests should be performed to ensure the initial performance conforms with the manufacturer’s specifications. Simultaneously, reference tests should be carried out to provide data against which the subsequent performance can be assessed by routine testing. Finally, daily operational checks should be implemented and records of the results of these tests should be kept as they may reveal unsatisfactory performance and enable the operator to take corrective actions. A successful QC scheme requires a clear definition of responsibilities, strict adherence to test frequency and protocols, and proper facilities for the follow-up of test results.
Quality control gives the users of an instrument confidence that it is operated in the best possible working condition. While QC may show that a failure has occurred, it rarely provides the exact diagnostic information needed for repair. Nevertheless, regular quality control testing is vital to the continuing satisfactory performance of any instrument.
The IAEA technical document “Quality control of nuclear medicine instruments” published in 1991 provides very detailed information about proper quality control for Nuclear Medicine instruments. In the IAEA Human Health Series No.33 “Quality Management Audits in Nuclear Medicine Practices” a checklist for quality control testing can be found. In addition, the IAEA book on Nuclear Medicine Physics gives a comprehensive overview on Nuclear Medicine in general.
IAEA-NMQC Toolkit
Free ImageJ plugins to support nuclear medicine physics equipment quality control examinations.
Proper quality control (QC) procedures in single-photon emission computed tomography (SPECT) play an essential role in ensuring optimized performance of imaging equipment, and are a requisite for providing high quality clinical care. Since QC procedures are implemented routinely, streamlining them with automated software may offer several benefits: improved speed, reduced errors, and the standardization of calculation methods across centres.
The IAEA Nuclear Medicine Quality Control (NMQC) Toolkit is a set of ImageJ (Fiji edition)-based codes developed in Java v.1.8 that allow the processing and analysis of nuclear medicine images acquired for quality control tests of gamma cameras and SPECT systems. Image acquisition of the tests should be performed as described in the guidance document IAEA Human Health Series No. 6 on “Quality Assurance for SPECT Systems” and/or in the NEMA NU 1-2012 standard - Performance Measurements of Gamma Cameras. Tutorial videos demonstrating in practice the procedures to perform the tests can be found here.
The IAEA-NMQC Toolkit has been developed to support common SPECT QC data analysis procedures for several QC tests. The plugins included in the IAEA-NMQC Toolkit are designed to be easy to use and allow the automatic or semi-automatic analysis of the following tests:
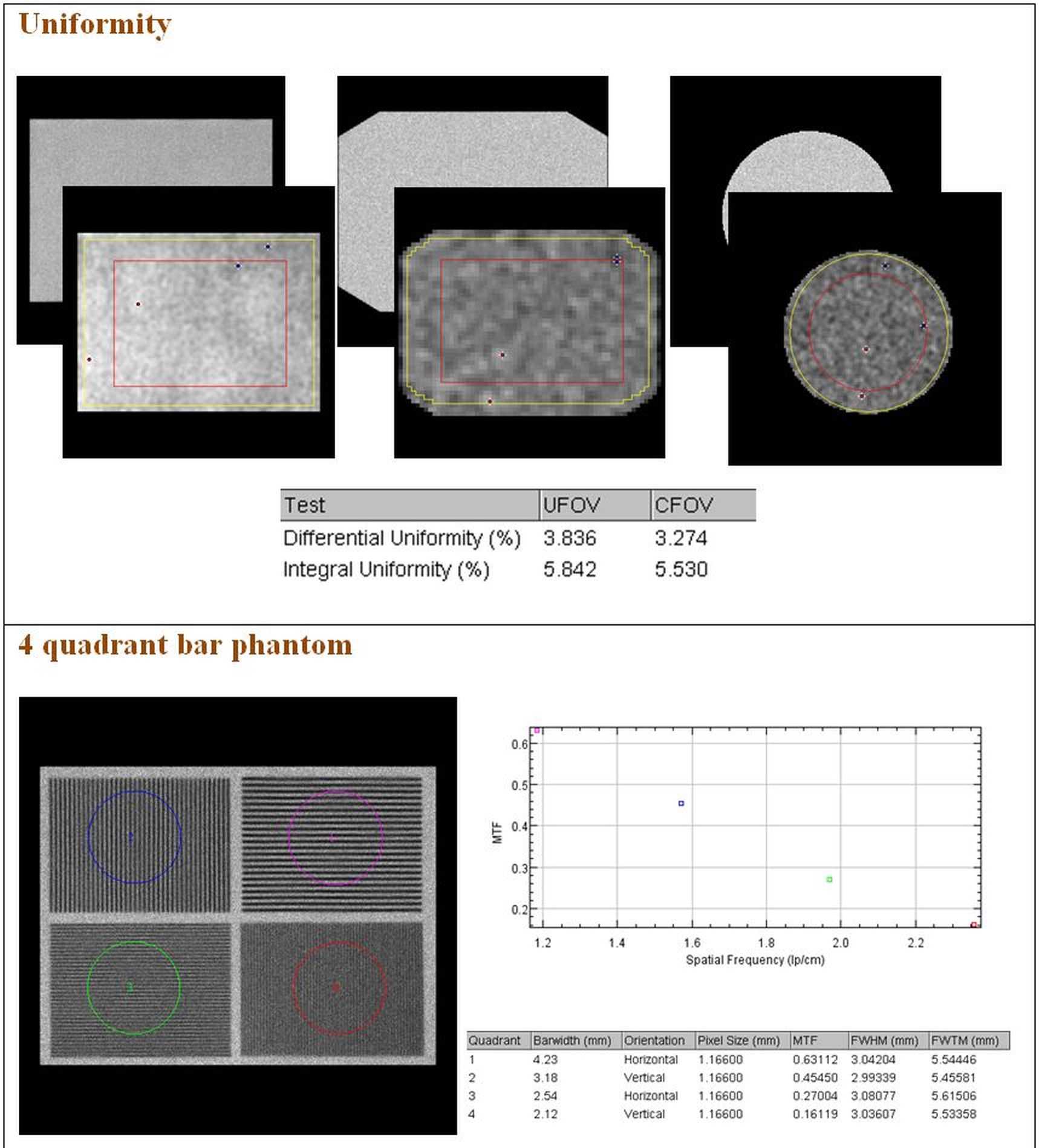
- Planar uniformity;
- Maximum count rate;
- Sensitivity;
- Intrinsic spatial resolution;
- Intrinsic spatial linearity;
- System spatial resolution;
- Pixel size;
- 4 quadrant bar phantom;
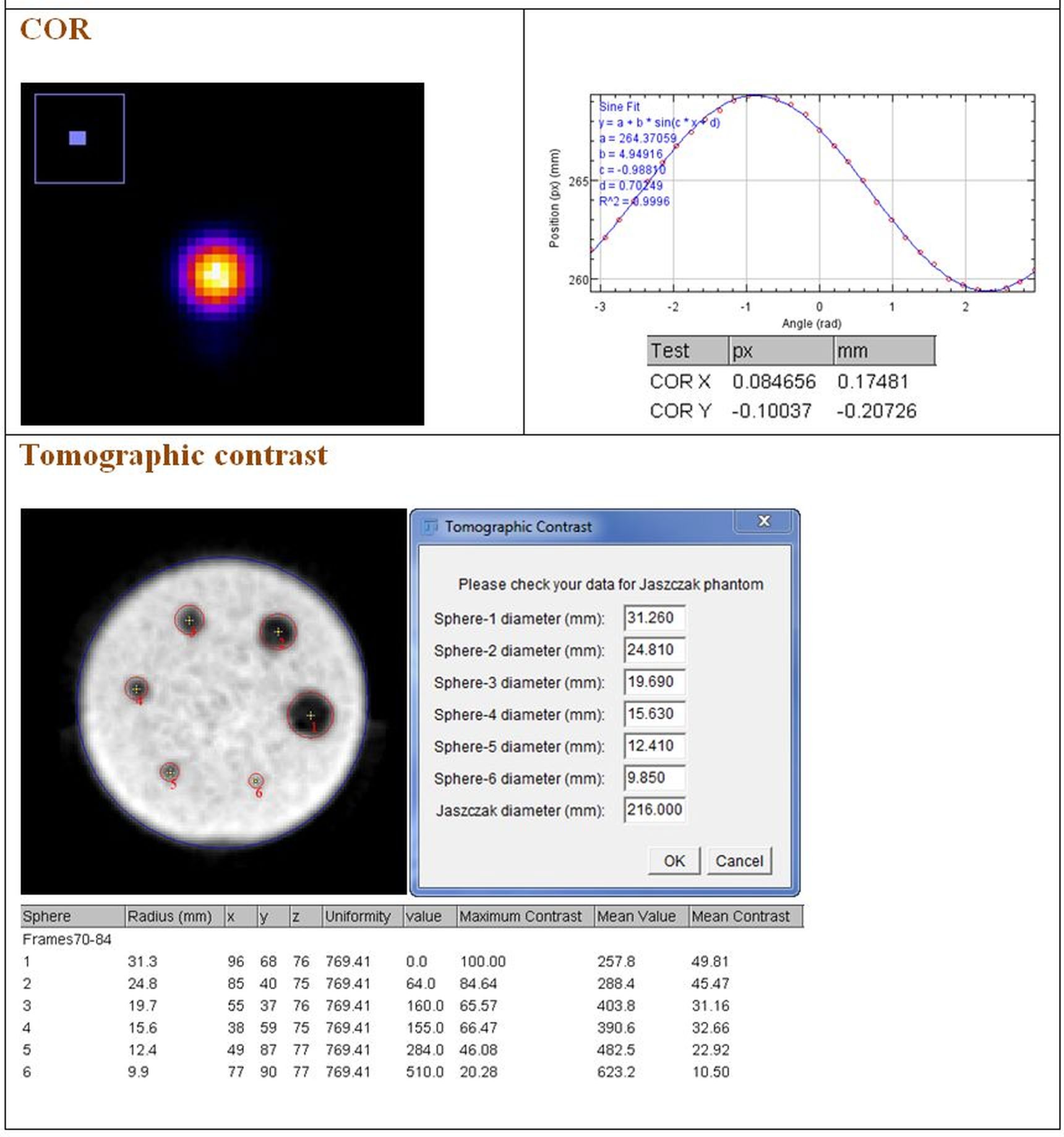
- Centre of rotation;
- Tomographic contrast;
- Tomographic resolution;
- Tomographic uniformity.

Installation instructions:
The IAEA-NMQC Toolkit is a free resource for the community to aid with standardized, rapid analysis of QC test images from various vendors.
To install:
- Install Fiji edition of ImageJ (downloadable here);
- Download IAEA-NMQC Toolkit here ;
- Copy the NMCQ_1.0.jar file to the “Plugins” folder of the Fiji installation.
Once these steps are completed, the NMQC plugins should be visible on the Fiji interface in the “Plugins” menu.
Detailed instructions for installation of IAEA-NMQC Toolkit and its use can be found in this User’s Manual.
The plugins included in the IAEA-NMQC Toolkit have been tested and validated using images acquired on machines frommajor vendors and/or using simulated images. Comments or suggestions are welcome, please send them by email to dosimetry@iaea.org.
A set of simulated images can be downloaded here
Below are some examples of the outputs given by the plug-ins.
Acknowledgements
The IAEA-NMQC Toolkit has been developed by Alex Vergara Gil (Division of Clinical Research, Centre of Isotopes, Havana, Cuba) with support and validation by Leonel Torres Aroche (Clinical Research, Centre of Isotopes, Havana, Cuba) and Gian Luca Poli (Dosimetry and Medical Radiation Physics section, Division of Human Health, IAEA).
Acknowledgement is also given for the assistance of Elena De Ponti (Ospedale San Gerardo, Monza, Italy), Adam Kesner (Memorial Sloan Kettering Cancer Center, New York, USA) and Federica Fioroni (Azienda Unità Sanitaria Locale – IRCCS di Reggio Emilia, Italy, Italy) for their help in acquiring the data and for the useful discussion, and of Theresa Feddersen and Elisabeth Salomon for their support in the validation.
The development of the IAEA-QCNM Toolkit was supported by the Peaceful Uses Initiative (PUI) project of Switzerland.
The IAEA officer responsible for the module was Gian Luca Poli, Dosimetry and Medical Radiation Physics section, Division of Human Health.


