Why is it useful to store the data in the template?
- In all organ level dosimetry work, biodistribution information must be recorded
- Template provides standardized format
- Ready-to-use and scales to support large biodistribution/dosimetry databases
- Enables easy quality control checks of data (e.g. quick review may ensure no more than 100% of activity is applied in dose calculations)
- Template can be shared for easy dissemination of work/results
- Acquiring biodistribution measurements can involve significant efforts to achieve. They are arguably more informative than dosimetry calculations and can be shared with the community
- Template is easily accessible. It can be viewed using freely available text editors, and easily parsed by user or commercial software
- Assumptions that affect dosimetry results – interpolation methods, phantom assumptions, mass scaling, etc. - can be easily studied/adjusted for analysis
- Template data can be reprocessed retrospectively
- Large data set can be collected and processed to support the study of populations with minimal user effort
- Dosimetry methodology/calculations can be shared, understood, and compared between groups/institutions
- The standardized way of describing data in the template may support advances in dosimetry software tools
- Data collection tools and procedures may be streamlined to output information in this standard format
- Users may write programs to automate dosimetry calculation processes that can be applied to populations, run fast, and reduce operator input error
- Users may write programs to advance dosimetry methodology (for example propagating error, visualization tools for biodistribution, studying dose in populations)
- Users may readily disseminate the related software they write to the community, to enable increased impact
- Vendors may build dosimetry calculation tools that readily integrate with varying data acquisition methods
Specifications
The IAEA Radiotracer Biodistribution Template is a structured format file that can be used to store specific radio-pharmaceutical biodistribution measurements for a subject, as well as the meta-details that explain how it was created and how it can be used.
The template is distributed in two file types .xlsx or .csv. The Excel version is available for users to enter data in a color formatted sheet (easier for organization), but we strongly encourage the completed distribution data should be saved/distributed in the .csv format. The .csv files are simple in structure, and can be used for automated code.
The files can be edited using standard spreadsheet programs such as MS Excel (freely available as Excel Online), or freely available Google Documents. Users may also write their own programs to parse the files.
Saving and disseminating
The suggested filename for saving completed templates is:
institution_radiopharmaceutical_ year_subjectID.csv
Files should be saved as type .csv for dissemination.
Users may distribute dosimetry data by including it as supplementary material in publications. Alternatively, users may post their data in on scientific data sharing sites - for example ResearchGate.net is a free website that allows users to post their raw data, making it accessible to the community.
The open access of biodistribution data (recorded in the IAEA Radiotracer Biodistribution Template) to users in the community may amplify the impact of work. If you post research data publicly, please let us know by emailing us. This will help us gauge how the sheet is being used, and we will try to collect this information and have it posted on our website.
Template structure
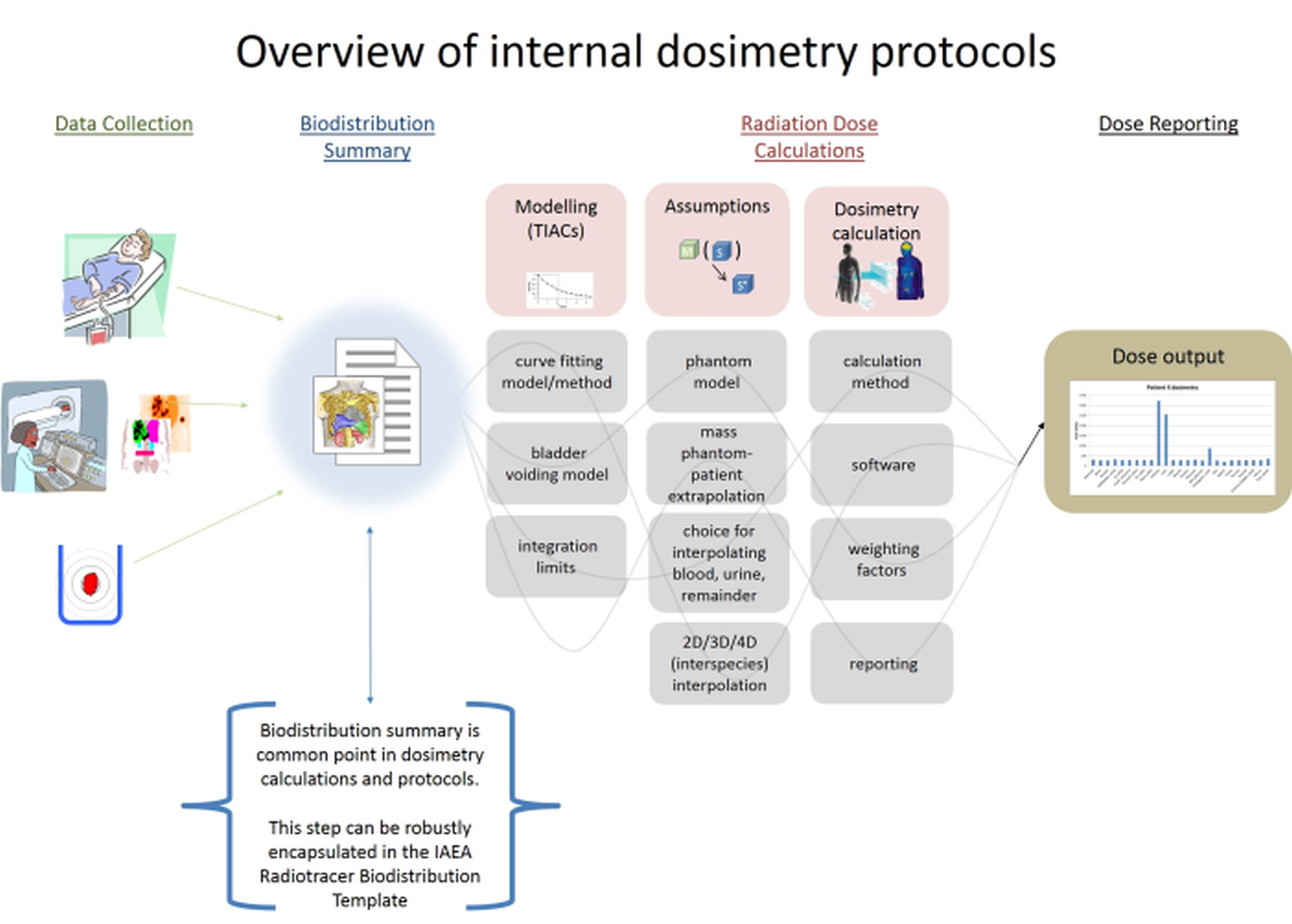
The IAEA Radiotracer Biodistribution Template is organized in information category blocks, as seen in the overview image below.
- Information about the type of study, technical details, and units for the presented data are defined in SECTION B and SECTION C.
- Raw data is contained in SECTION D.
- Time-Integrated Activity Coefficients are contained in SECTION E.
- Comments clarifying the details of the acquisition/data can be included in SECTION F.

How to use/complete the template
The IAEA Radiotracer Biodistribution Template is meant to document radionuclide biodistribution measurements. This template does not contain dosimetry calculations. However, the intention is that the basic information recorded here may be used for further organ level dosimetry calculations.
To complete the template, enter single field details, then measurement time, volume, activity, and standard deviation, per organ, for the organs that have this information available.
All editable fields initially contain an empty flag (-1). These fields should be changed only when information is available to complete them. It is acceptable/expected that not all fields will be modified. For example, fields delineating organs which are not measured should be left with an empty flag (-1). Measurements only need to be entered for assessed organs/time points.
The template is structured into the following sections:
- SECTION A - TEMPLATE DESCRIPTION
- Instructions for template use
- SECTION B - ACQUISITION DETAILS
- Enter details describing the acquisition of input data
- Fields include: Subject ID, Species, Sex, Height, Weight, Projected whole body blood volume, Isotope, Radiopharmaceutical, Date of administration, Time of administration, Net injected activity, Software used for image analysis, Institution of acquisition, City of acquisition, Author initials (optional), Inquiry contact email (optional).
- SECTION C - TECHNICAL DETAILS
- Enter details describing technical aspects of data acquisitions
Main measurement modality, Blood measurement modality, Blood measurement units, VOI/ROI measurement units, Organ VOI/ROI drawing method, Time point units, Volume units, Method of calibration, Attenuation correction applied, Geometric mean applied, Decay correction applied, Scatter correction applied, Partial volume correction applied, Well Counter Calibration Factor, Probe Calibration Factor, SPECT Calibration Factor, Time integrated activity coefficient (residence time)
- SECTION D1 - MEASUREMENT TIME POINTS
- Enter time points associated with the activity measurements used in Section D3
- For each measurement that will be recorded in section D3, an associated time point needs to be entered
- Units are specified in section C (suggested hours post injection)
- SECTION D2 - MEASUREMENT VOLUMES
- Enter organ volume, specific to subject studied, for each measurement taken
- Units are specified in section C
- SECTION D3 - MEASUREMENT ACTIVITIES
- Enter activity measured for each organ/time point studied
- Units are specified in section C
- SECTION D4 - MEASUREMENT STANDARD DEVIATIONS
- If available, enter the standard deviations associated with the measurements in section D3
- SECTION E - Time-Integrated Activity Coefficients (TIACs) (residence times) (OPTIONAL)
- User may optionally include TIACs calculations derived in section E
- Time-Integrated Activity Coefficients can be entered with units of hours, or total number of disintegrations, or % of total disintegrations. This detail is specified by user in SECTION C
- SECTION F – COMMENTS
- The comments section has several categories. Please include any comments that would be relevant for an objective reviewer to better understand the study
Examples
Example completed templates can be downloaded for Lu-177 DATATATE, I-131.
Types of dose calculation
The IAEA Radiotracer Biodistribution Template supports organ-level dosimetry, which means that activities are assumed to be uniform within organs and the emissions from that organ can be summarized with a single number (i.e. Time-Integrated Activity Coefficients). Dose calculations are usually calculated from the organ-level biodistribution (recorded in the template) using cross-organ dose factors and the MIRD formalism. The cross-organ dose factors are referred to as “S factors” in the MIRD formalism, or “dose conversion factors” in the OLINDA/EXM software, and are calculated for specific digital phantoms and nuclides and summarize the dose deposited from emissions from one organ to another.
There are alternative methods for calculating internal dosimetry, for example Monte Carlo methods, or dose point kernel methods. Users may explore literature to learn more about these alternative methods.
Advancements in dosimetry methodology based upon use of this template
Many aspects of dosimetry collection would benefit from improved standardization. This template is free for the community and will remain that way. Users who develop tools or publish improvements to dose calculation procedures are encouraged to share their efforts by emailing dosimetry@iaea.org.
Utilization of template for generalized code
The template may be updated in the future, depending on community input. While changes may occur, care will be taken to keep the three letter UID tags (3rd column in sheet) consistently associated with its respective variable. Thus it is recommended to use those tags for building parsing software.
Feedback
We are presenting this template as a resource for the community. We invite feedback on this effort. Please send us comments at dosimetry@iaea.org.
An overview of dosimetry principles can be found on the IAEA human health campus website, in the nuclear medicine dosimetry section.
Acknowledgement
The IAEA Radiotracer Biodistribution Template has been developed by Adam Kesner, PhD, University of Colorado, Denver, CO, USA and Michael Lassmann, PhD, Universitätsklinik Würzburg, Würzburg, Germany with support by Seval Beykan, Universitätsklinik Würzburg, Würzburg, Germany, and Gian Luca Poli, IAEA.