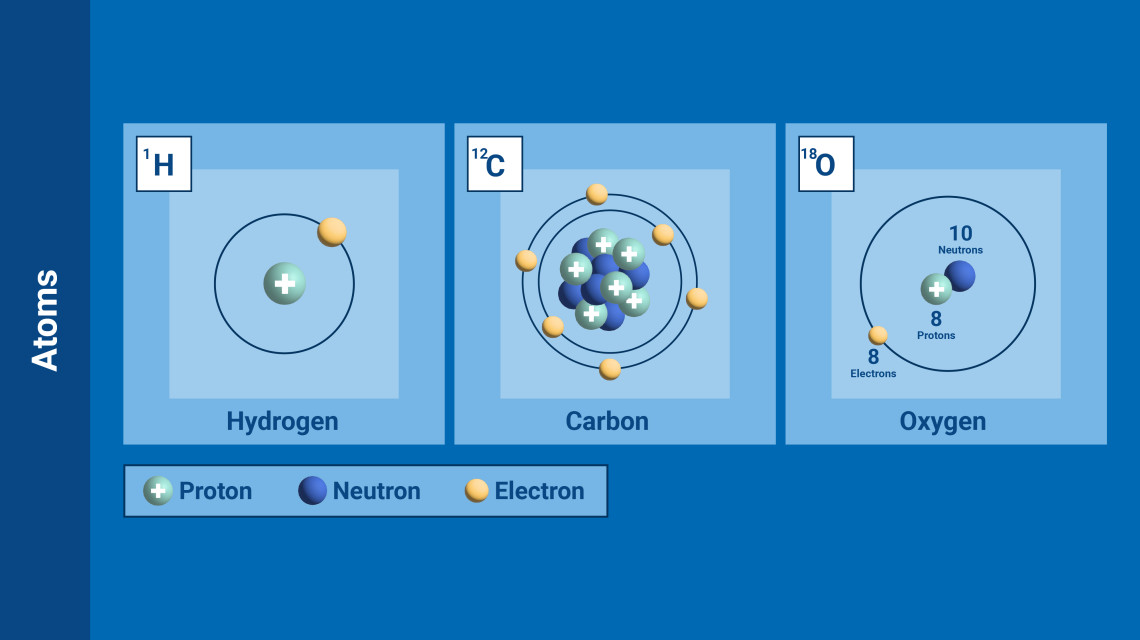
(Graphic: A. Vargas/IAEA)
Each element is distinguished by the number of protons, neutrons and electrons that it possesses. The atoms of each chemical element have a defining and same number of protons and electrons, but – crucially – not neutrons, whose numbers can vary.
Atoms with the same number of protons but different numbers of neutrons are called isotopes. They share almost the same chemical properties, but differ in mass and therefore in physical properties. There are stable isotopes, which do not emit radiation, and there are unstable isotopes, which do emit radiation. The latter are called radioisotopes.












