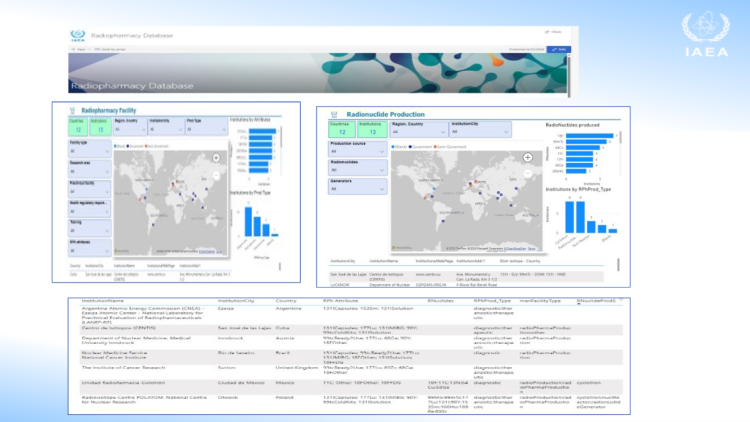
A new IAEA radiopharmacy database will facilitate research, collaboration and the sustainability of safe radiopharmaceuticals for clinical use. The IAEA is currently collecting information on facilities that produce radiopharmaceuticals, as well as those producing medical radioisotopes for use in radiopharmaceuticals worldwide, to be collated into the new online database. The database aims to create greater insight into radiopharmaceutical trends. It will highlight any supply gaps and facilitate connections among the different producers of these products, improving access to radioisotopes and radiopharmaceutical products in such facilities.
Radiopharmaceuticals are a special class of pharmaceutical products that typically have a short shelf life, ranging from a few hours to a few days. This limits their wider distribution and makes it essential to have the appropriate production facilities readily available. Radiopharmaceuticals are produced in various settings, including hospitals, centralized radiopharmacies and industrial radiopharmacies, before being distributed for use.
Find out more: what are radiopharmaceuticals?